Minecraft PC IP: play.cubecraft.net
So please just stop already
edit: o it's someone's alt löl
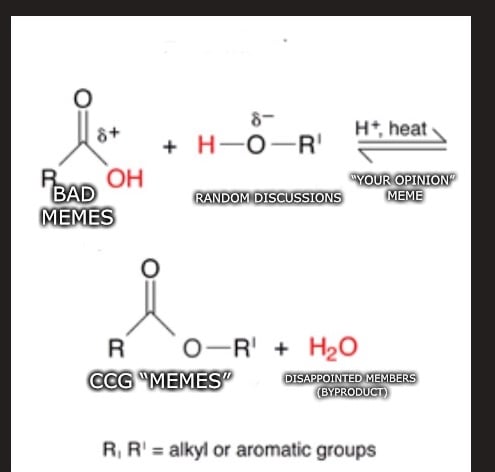
then it decomposes back in a miniute
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
CCG Memes
- Thread starter Muffin
- Start date
Memes about tryharders do have a connection with cube.Your "no u" tennis meme has absolutely no connection with cube?
What about not criticizing memes and just enjoy them. It's CubeCraft related, it's about tryharders. If this meme isn't CCG related tons of memes aren't either. You're going to comment on all of those either? Yeah I didn't think so. So please just stop already.Your "no u" tennis meme has absolutely no connection with cube?
What about reading the original thread where the "rules" are explainedWhat about not criticizing memes and just enjoy them. It's CubeCraft related, it's about tryharders. If this meme isn't CCG related tons of memes aren't either. You're going to comment on all of those either? Yeah I didn't think so. So please just stop already.
So please just stop already
What about reading the original thread where the "rules" are explained
So please just stop already
Tryharders are related to ccgn, so I don't know what's your problem.Throw out your best memes that're created by you and are related to CCG.
There was no problem until SOMEONE had to be super agressive :shrug:Tryharders are related to ccgn, so I don't know what's your problem.
edit: o it's someone's alt löl
Nobody cares or wants to see your idiotic conversation, go to CCG Discussions.
Did I miss something? (Let's move to ccg discussions okay)There was no problem until SOMEONE had to be super agressive :shrug:
is there smth like that lolNobody cares or wants to see your idiotic conversation, go to CCG Discussions.
D
Deleted member 352903
Guest
C
Cutee
Guest
That's your opinion ¯\_(ツ)_/¯
It just died.Just how long did this thread stay alive?
then it decomposes back in a miniute
The then it decomposed back in a minute part is not how chemistry works. The sign that allows it to go back and forth actually means the reaction will change back as soon as enough of the reactants are accumulated. Eventually the reaction will reach equilibrium where proportionally there will be proportionally the same amount of reactants and products. This takes into account the amount of heat, quantity of the items and the pressure if there is gas involved in the reaction.View attachment 147420
then it decomposes back in a miniute
Similar threads
- Replies
- 7
- Views
- 575
- Replies
- 3
- Views
- 452
Latest profile posts
NEWS:
1 - Chicken Jockey Buddy released yesterday
2 - Bundle and Maps Notion post has been updated to change emojis from a Monkey to a shush face emoji

1 - Chicken Jockey Buddy released yesterday
2 - Bundle and Maps Notion post has been updated to change emojis from a Monkey to a shush face emoji




